PHOTOSYNTHETIC PIGMENTS
🌱 Comprehensive Notes on Photosynthetic Pigments
🌞 What are Photosynthetic Pigments?
Definition: Molecules that absorb light energy and transfer it to the photosynthetic machinery for conversion into chemical energy.
Role:
- Broaden the absorption spectrum.
- Capture solar radiation.
- Protect photosystems from excess light damage.
TABLE:1 Photosynthetic Pigments, their distribution & absorption peaks
|
Pigment |
Distribution in Plant Kingdom |
Absorption Peaks (nm) |
|
Chlorophyll a |
Universal in all oxygenic photosynthetic organisms (plants, green algae, red algae, cyanobacteria) |
430–435 nm (blue), 662–665 nm (red) |
|
Chlorophyll b |
Green algae, higher plants (absent in cyanobacteria, red algae, brown algae, diatoms) |
453–460 nm (blue), 642–650 nm (red) |
|
Chlorophyll c (c1, c2, c3) |
Brown algae, diatoms, dinoflagellates, chrysophytes |
447–452 nm (blue), 580–585 nm (red-orange) |
|
Chlorophyll d |
Certain red algae, cyanobacteria |
710 nm (far-red) |
|
Chlorophyll f |
Cyanobacteria (specialized, found in shaded/infrared environments) |
720–740 nm (infrared) |
|
Carotenoids (β-carotene, lutein, violaxanthin, etc.) |
Widely distributed in higher plants, green algae, many other algae, cyanobacteria |
400–500 nm (blue-violet) |
|
Xanthophylls (oxygenated carotenoids: lutein, zeaxanthin, fucoxanthin, peridinin) |
Lutein in higher plants and green algae; fucoxanthin in brown algae & diatoms; peridinin in dinoflagellates |
450–540 nm (blue-green) |
|
Phycobilins (phycoerythrin, phycocyanin, allophycocyanin) |
Red algae, cyanobacteria (organized in phycobilisomes) |
Phycoerythrin: 495–570 nm (green-yellow); Phycocyanin: 610–620 nm (orange-red); Allophycocyanin: 650–655 nm (red) |
📈ABSORPTION SPECTRA
The visible part of the spectrum of electro-magnetic radiation (that ranges from wavelength 390 nm to 760 nm) which is absorbed by the photosynthetic pigments of organisms, is called absorption spectra.
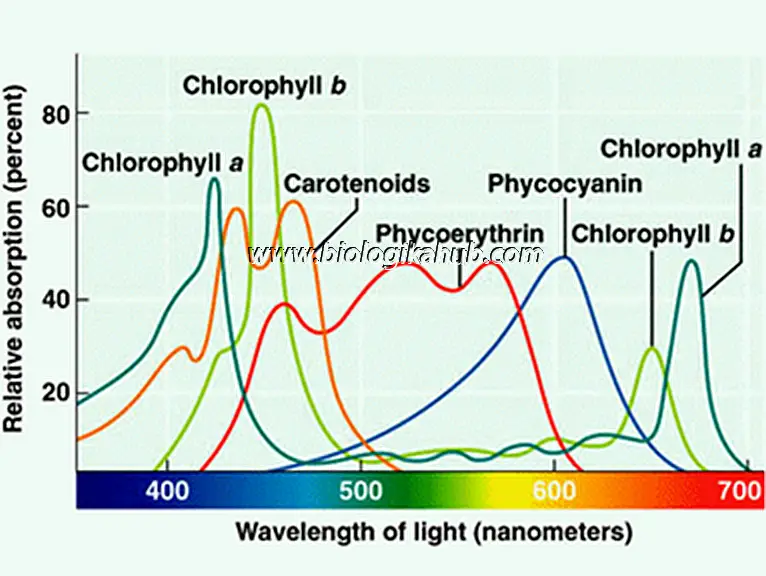
Figuer 1: Absorption spectrum of the photosynthetic pigments showing their respective range of absorption of different wavelengths of light
🍀CHLOROPHYLL PIGMENTS🍀
Chlorophyll a
Chemical Formula → C₅₅H₇₂O₅N₄Mg
Molecular Structure →
Porphyrin ring with magnesium (Mg²⁺) at the center
Long phytol tail (hydrophobic, anchors pigment in thylakoid membrane)
Contains a methyl group (-CH₃) at C3 position
Functions →
Primary photosynthetic pigment
Present in reaction centers P680 (PSII) and P700 (PSI)
Directly converts light energy into chemical energy
Chlorophyll b
Chemical Formula → C₅₅H₇₀O₆N₄Mg
Molecular Structure →
Similar porphyrin ring with Mg²⁺
Phytol tail
Contains an aldehyde group (-CHO) at C3 position instead of –CH₃
Functions →
Accessory pigment in higher plants, green algae
Broadens absorption spectrum by capturing blue light
Transfers captured energy to chlorophyll a
TABLE:2 Differences between Chlorophyll a and Chlorophyll b
|
Feature |
Chlorophyll a |
Chlorophyll b |
|
Chemical formula |
C₅₅H₇₂O₅N₄Mg |
C₅₅H₇₀O₆N₄Mg |
|
Side group at C3 |
Methyl (-CH₃) |
Aldehyde (-CHO) |
|
Reflecting Color |
Blue-green |
Yellow-green |
|
Absorption peaks |
430–435 nm (blue), 662–665 nm (red) |
453–460 nm (blue), 642–650 nm (red) |
|
Function |
Primary pigment (reaction center) |
Accessory pigment (light harvesting) |
|
Occurrence |
Universal in all oxygenic photosynthesizers |
Only in green algae & higher plants |
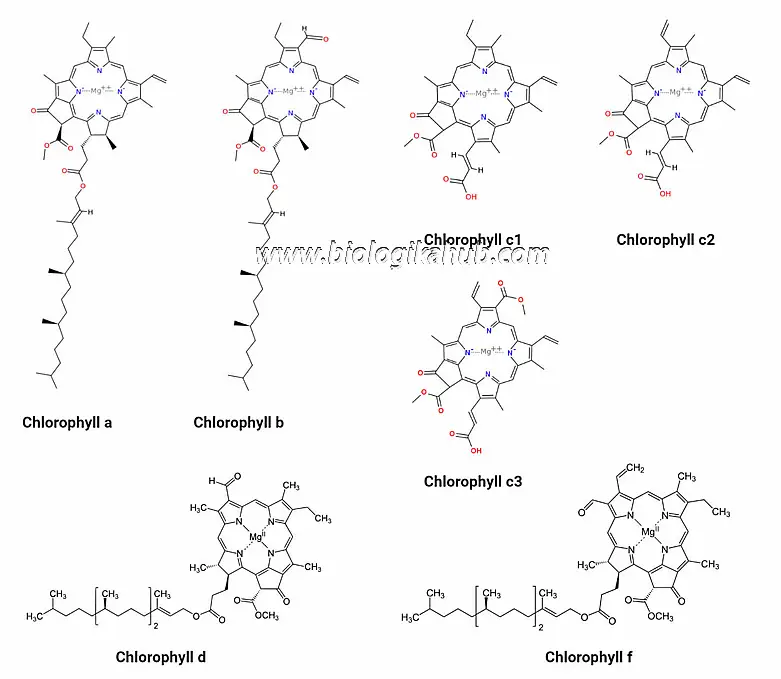
Chlorophyll C
Chemical Formula → C₃₅H₂₈O₅N₄Mg
Molecular Structure →
Porphyrin ring with Mg²⁺
Lacks phytol tail (unlike chlorophyll a & b) → makes it more polar
Exists as c₁, c₂, c₃ variants
Functions →
Accessory pigment in brown algae, diatoms, dinoflagellates, chrysophytes
Broadens light absorption (blue-green region)
Transfers energy to chlorophyll a
Chlorophyll d
Chemical Formula → C₅₄H₇₀O₆N₄Mg
Molecular Structure →
Similar to chlorophyll a but with a formyl group (-CHO) at C3 position of ring A
Long phytol tail for membrane anchoring
Functions →
Found in some red algae and cyanobacteria (Acaryochloris marina)
Absorbs far-red light (~710 nm) → adaptation to low-light aquatic environments
Transfers energy to chlorophyll a in reaction centers
Chlorophyll f
Chemical Formula → C₅₅H₇₀O₆N₄Mg
Molecular Structure →
Similar porphyrin structure with Mg²⁺
Has a formyl group (-CHO) at C2 position of ring A
Long phytol tail present
Functions →
Discovered in cyanobacteria (2010, Australia)
Absorbs infrared light (720–740 nm) → allows photosynthesis beyond visible light
Helps organisms survive in deep-shaded or infrared-rich environments
🥕CAROTENOID PIGMENTS🥕
Carotenoids are natural pigments found in plants, algae, and some bacteria and fungi, responsible for the yellow, orange, and red colors of many fruits and vegetables.
Light Absorption & Color →
Absorb 400–550 nm (violet to green light)
Cause bright yellow, orange, red colors
Dominant in autumn leaves of 15–30% tree species
Structure →
Tetraterpenoids → 40 carbons (from 4 terpene units of 10C each)
Polyene hydrocarbon chain with 9–11 double bonds, may end in rings, may ± oxygen atoms
Conjugated double bonds → high reducing potential
Able to transfer electrons
Polyene hydrocarbon chain,
Usually lipophilic (long unsaturated chains)
Electron Transfer Mechanisms →
Singlet–Singlet transfer: carotenoid → chlorophyll (photosynthesis)
Triplet–Triplet transfer: chlorophyll → carotenoid (photoprotection, ROS control)
Types of Carotenoids →
Carotenes: unoxygenated hydrocarbons (α-carotene, β-carotene, lycopene)
Xanthophylls: oxygenated carotenoids (lutein, zeaxanthin)
🍂Carotenes
Unoxygenated (oxygen-free) carotenoids composed of only carbon and hydrogen
Empirical formula: C40H56
Colors: orange, red
Examples: α-carotene, β-carotene, lycopene
(β-carotene is the most common carotene. It’s both ends are cyclicised. It absorbs light mostly between 400-500 nm.
Lycopene is carotene found in tomato fruit. It has open ends).
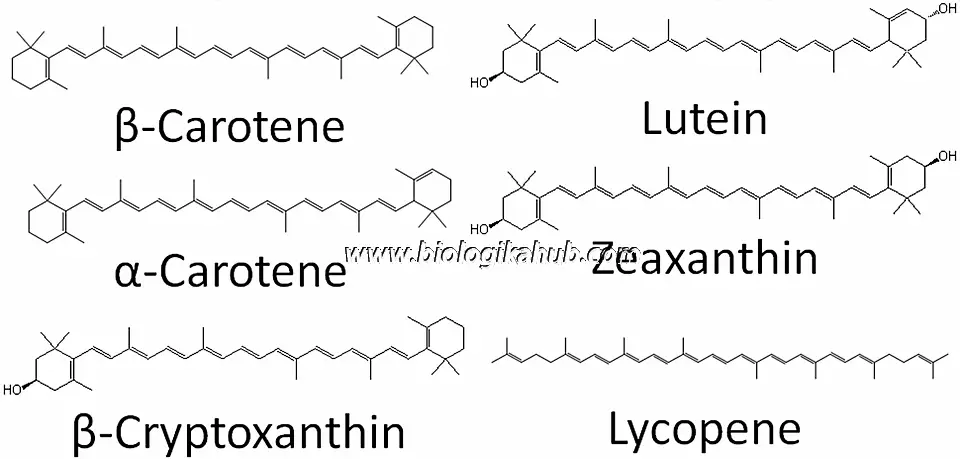
🍁Xanthophylls
Yellow-colored oxygenated carotenes
Empirical formula: C40H56Oₓ (x = 1–8 oxygen atoms)
Examples: Lutein → hydroxylated α-carotene, Zeaxanthin → hydroxylated β-carotene
🧠Mind Dumps (Quick Recall Tools)
⚡ Core Pigment Facts
Chl a = universal, primary pigment.
Chl b = accessory in plants/green algae.
Chl c, d, f = specialized adaptations.
Carotenoids = photoprotection + accessory absorption.
Phycobilins = aquatic advantage pigments.
🎯 Mnemonic for Chlorophyll Variants
“Always Be Clever During Finals” → a, b, c, d, f.
⚡ Absorption Peaks Quickie
Chl a → Blue 430 nm, Red 662 nm.
Chl b → Blue 453 nm, Red 642 nm.
Carotenoids → Blue-Violet 400–500 nm.
Phycobilins → Green–Red 495–655 nm.
🍁
